Starting Your Research
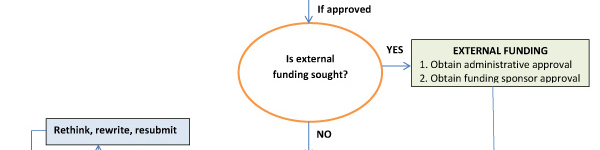
According to the NUHS policy “Research Proposals,” all research projects, regardless of subject matter or funding source, must be reviewed for scientific merit, time commitment, and funding requests to ensure that the research is of the highest quality possible. Before starting a project, complete and submit the Application for Approval of a New Research Project to the NUHS Research Committee for review.
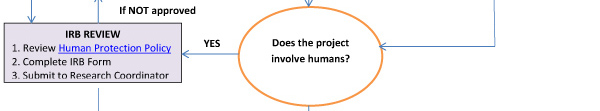
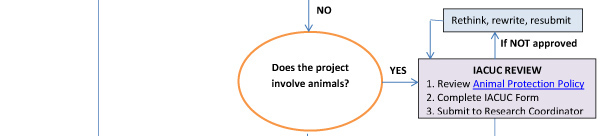
Research utilizing human or animal subjects requires subsequent review for ethical consideration. For additional information on preparing your application, see Guide for Preparations and Processing of Human Research (The Blue Book).